Abstract
Background: Detection of measurable residual disease (MRD) after Hematopoietic Stem Cell Transplantation (HSCT) is an early predictor of frank relapse. The role of DLI as a pre-emptive treatment to prevent relapse is still debated. Blinatumomab, a CD3/CD19 bispecific T-cell engager molecule, is approved in adult patients with MRD and in children with relapsed or refractory acute lymphoblastic leukemia (ALL).
Aims: We evaluated the safety and feasibility of blinatumomab plus Donor Lymphocyte Infusion (DLI) to treat MRD-positive pediatric ALL after HSCT. Here, we report our experience in three patients who were MRD-positive post-allogenic HSCT (allo-HSCT) for ALL and received blinatumomab and DLI.
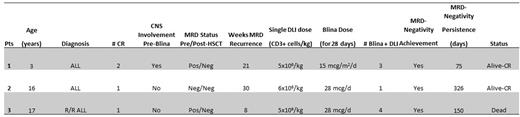
Methods: Three pediatric patients with high-risk B-ALL all were treated with blinatumomab plus DLI for MRD relapse after HSCT. Patients' characteristics are summarized in the Table.
Results: Patient 1, a 3- year old child, developed Central Nervous System (CNS) relapse during maintenance, then received salvage therapy according to the INTREALL HR 2010 and 1 course of blinatumomab, achieving MRD-positive Complete Remission 2 (CR2). Patient 2, a 16- year old female, had secondary ALL and achieved CR1 after 1 course of chemotherapy. She subsequently received 2 courses of blinatumomab, due to chemotherapy-associated toxicity and achieved MRD-undetectable CR1. Patient 3, a 17-year old male, was primary refractory to 2 lines of chemotherapy and blinatumomab. He received 2 courses of inotuzumab and achieved MRD-positive CR. All patients proceeded to allo-HSCT and were MRD-positive at 21, 30 and 8 weeks post-allo-HSCT, respectively. All patients discontinued immunosuppressive therapy and received 3, 1 and 4 courses of DLI plus blinatumomab, respectively. Blinatumomab was started one week after each DLI and was given at a dose of 15 mcg/m2/d or 28 mcg/d, according to the patients' age, for a total of 4 weeks each course. Only patient 3 experienced Grade 2 liver GVHD. None of the patients experienced serious adverse events requiring blinatumomab discontinuation. Patient 1 achieved MRD-negative CR after two courses of combined treatment, subsequently he experienced CNS relapse, treated with intrathecal chemotherapy and radiation, reaching a CNS and Bone Marrow-MRD negative CR. Patient 2 achieved MRD-undetectable CR and is still in MRD negative CR. Patient 3 developed isolated CNS relapse after 4 courses of combined DLI plus blinatumomab. Patient was treated with radiation and Intrathecal chemotherapy, reaching a CNS negative and Bone Marrow-MRD negative CR. Patient died for unknown complications.
Conclusions: The role of cellular therapy with DLI is still debated in ALL. Most post-transplant relapses are associated with loss of surface HLA by the leukemia cells, hence DLI alone has limited efficacy in patients with post HSCT relapse. Blinatumomab directs T cells to bind CD19 present on malignant B cells engaging CD3 on T cells and inducing activation and cytotoxicity against ALL blasts. We, therefore, tested the efficacy of blinatumomab plus DLI in 3 patients with MRD-positive ALL after allo-HSCT. The use of blinatumomab allowed recruitment of fit donor-derived T lymphocytes (not exposed to immune suppressive agents) against ALL B cells. This hypothesis is supported by the fact that patient 3, who received blinatumomab pre-HSCT and had disease progression, probably due to lack of T cells, as showed by flow cytometry, achieved MRD-undetectable status after receiving DLI plus blinatumomab post-transplant. Moreover, two patients reached stable MRD-undetectable status and 2 subsequently developed CNS relapse; none received CNS prophylaxis post-HSCT. We hypothesize that blinatumomab plus DLI can clear the hematologic disease, but it is not effective in preventing CNS relapse. Although very interesting, this hypothesis should be confirmed in a larger cohort of patients.
Disclosures
Tambaro:Jazz: Other: Meeting participation fees, Speakers Bureau; Gilead: Speakers Bureau; Novartis: Other: Meeting Participation fees.
OffLabel Disclosure:
Blinatumomab is approved in adult patients with MRD and in children with relapsed or refractory acute lymphoblastic leukemia ALL.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal